1. a) An example of a chemical element is
b) When equal volumes are evaporated, the largest amount of solid will be produced by
distilled water river water sea water tap water
c) A gas which relights a glowing splint is
hydrogen methane nitrogen oxygen
d) A gas which causes rain to have a pH value of 5 is
carbon dioxide methane nitrogen oxygen
e) A substance which turns anhydrous copper sulphate from white to blue is
carbon dioxide ethanol petrol water
f) A substance not found naturally but made from a raw material is
coal polythene sand wood
g) A substance used by farmers to make their fields less acidic is
clay lime manure sand
h) A substance which cannot be used as a fuel is
coal methane water wood
i) A process which reduces the amount of carbon dioxide in the atmosphere is
burning fossil fuels burning wood photosynthesis respiration
j) Oxygen is used commercially for
filling airships keeping ice cream making steel putting out fires
(10)
2.
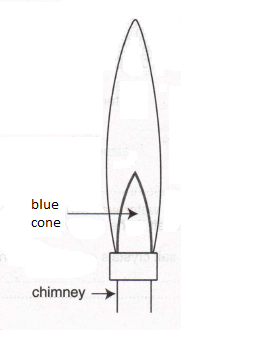
(a) Label, with an X, the hottest part of the Bunsen flame shown above. (1)
(b) Would the air hole be open or closed to produce this flame?
(1)
(c) What would you do to reduce the height of this flame by about a half?
(1)
(d) What would you do to make the flame wavy and luminous?
(1)
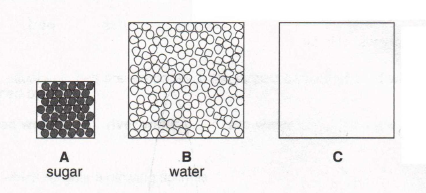
3. Look at these diagrams of the molecules in sugar and in water. In box C draw the molecules in sugar solution. (You need
not fill box C completely with molecules.)
4. In an experiment some sea water was evaporated over a hot water bath and small crystals of salt were left in the evaporating basin.
(a) Describe how larger salt crystals could be obtained.
(2)
(b) In the experiment,
(i) what is the solvent
(1)
(ii) what is the solute?
(1)
(c) Which word means that a substance changes from a liquid to a gas?
(1)
5. You may use each of the following once, more than once, or not at all to answer the questions which follow.
argon carbon dioxide hydrogen nitrogen oxygen
Which gas
(a) is there most of in air
(1)
(b) is needed for combustion
(1)
(c) do plants produce during photosynthesis
(1)
(d) ignites with a squeaky pop?
(1)
6. Write down the name of
(a) the light brown metal which turns black when heated in air
(1)
(b) a gas which burns with a blue flame to give water and carbon dioxide
(1)
(c) the black solid which collects on the outside of an evaporating basin when it is heated by a yellow Bunsen flame.
(1)
7. James made an indicator from a large red cabbage leaf. His instructions are written down below but in the wrong order.
(a) A Filter the mixture.
B Boil for ten minutes.
C Chop the leaf finely.
D Add 50 cm3 pure water.
Using the letters A, B etc, give the correct order.
(3)
(b) James tested the indicator on solutions of known pH and obtained these results.
(i) Which colour is the indicator in an alkaline solution?
(1)
(ii) Which colour is the indicator in a neutral solution?
(1)
(c) (i) Name a substance James could add to some red cabbage indicator to turn it red.
(1)
(ii) If James added some dilute sodium hydroxide solution, drop by drop, to the solution from (c)(i) until it was alkaline,
what colour changes would he see?
(2)
(iii) Explain what the sodium hydroxide is doing to the solution in (c)(i) and give the name we use for this type of
reaction.
(2)
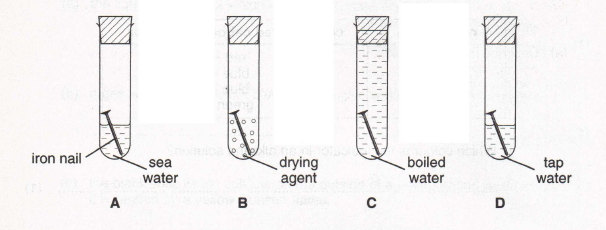
8. Simon set up an experiment to test the effect of sea water on rusting, as shown below.
Here are the results after three days.
(a) Why is there no rust in test tubes
(i) B (1)
(ii) C? (1)
(b) Simon has a new bicycle and plans to spend two weeks with it at the seaside. What might happen to some of the exposed metal parts made of iron?
(1)
(c) What advice would you give him to prevent this happening? Explain your answer.
(2)
9. For a chemistry project Sarah investigated the green solid which had formed on a copper roof at her school. She was given some scrapings of this solid, which she reacted with dilute sulphuric acid. It fizzed and dissolved to give a blue solution which she thought was copper sulphate.
(a) Describe how Sarah might have collected a few test tubes of the gas given off when the green solid was reacted with dilute sulphuric acid. You may find it helpful to draw a diagram in the space below.
(3)
(b) Describe a reaction Sarah could have done with the blue solution to extract metallic copper from it. Write a word equation for the reaction you have chosen.
(2)
Word equation:
copper sulphate + --> copper + (2)
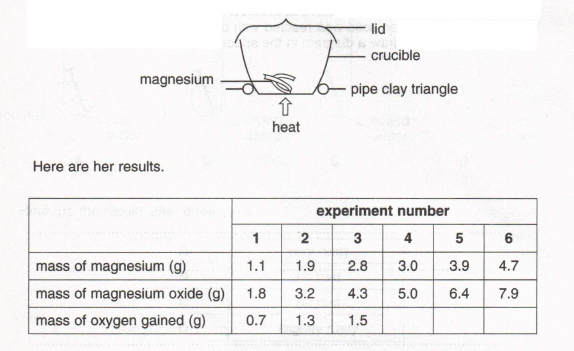
10. Christine did an experiment to measure the increase in mass when magnesium is heated in the air. She used the apparatus shown below and raised the crucible lid several times in each experiment.
(a) Complete her results table to show the mass of oxygen gained for the last three experiments
(1)
(b) Plot a graph of the mass of magnesium against the mass of oxygen gained. Draw the best straight line through the points.
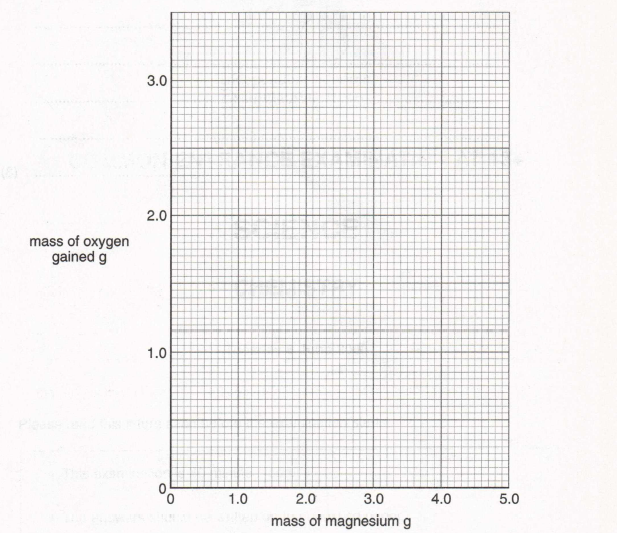
(c) Why did Christine raise the crucible lid several times in each experiment?
(2)
(d) What might have gone wrong in the third experiment?
(1)
(e) Describe how you would test the magnesium oxide and the magnesium to show that they are different chemical substances.
(3)