1 a) A substance having a fixed volume and shape would be a
b) Metals are always
dull solids electrical insulators good conductors of heat magnetic
c) When a solid turns to a gas without becoming liquid, the solid
condenses distils evaporates sublimes
d) In sea water, salt is the
solute solution solvent sublimate
e) A test for water uses
anhydrous copper sulphate copper oxide
indicator paper potassium manganate (VII)
f) The gas which makes up about 20% by volume of the atmosphere is
carbon dioxide hydrogen nitrogen oxygen
g) A substance which increases in mass when heated in air could be
copper copper carbonate copper oxide copper sulphate
h) The substance with the highest pH in this list is
dilute hydrochloric acid distilled water limewater vinegar
i) Pure salt crystals could be obtained from a mixture of sea water and sand by
evaporating all the water fractionally distilling the mixture
filtering the mixture and then evaporating the water
filtering the mixture and then removing the salt crystals from the filter paper
(9)
2. (a) To produce a yellow, sooty flame, does the airhole of a Bunsen burner have to be open, half open or fully closed?
(1)
(b) Complete the word equation for the reaction between aluminium and iodine.
aluminium + iodine ---> (1)
(c) Give one everyday use of neutralisation.
(1)
(d) Give one use for limestone.
(1)
3. The following are all used in the laboratory to separate substances:
chromatography crystallisation distillation evaporation filtration
Choose the one which would be test for
(a) separating the dyes in black biro ink (1)
(b) obtaining water from sea water (1)
4. This question concerns the elements
carbon copper Iron magnesium sulphur zinc
Select from the list
(a) two elements which form acidic oxides
(i) (1)
(ii) (1)
(b) an element which turns black when heated strongly in air
(1)
(c) the element which burns vigorously in air with a bright white flame
(1)
(d) an element whose oxide contributes to global warming
(1)
(e) the least reactive metal
(1)
(f) the element which does not conduct electricity
(1)
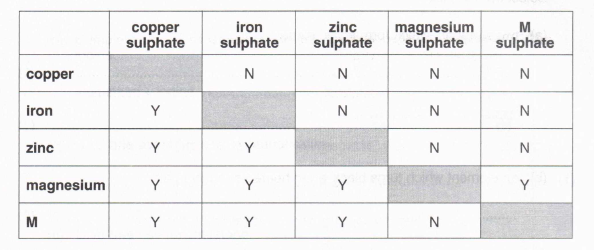
5. The table shows the results of some experiments to investigate the reactivities of copper, iron, zinc, magnesium and an unknown metal M. In the table, Y indicates that a reaction was observed and N that no reaction was observed when the metal was added to the solution of the metal sulphate shown.
(a) (i) Which metal is the most reactive? Explain your answer.
(3)
(ii) Write a word equation for the reaction which takes place when this metal is added to copper sulphate solution.
(1)
(iii) What would you expect to see in this reaction?
(2)
(b) Arrange magnesium, zinc and the unknown metal M in order of decreasing reactivity (most reactive first).
(1)
(c) (i) Which gas would you expect to be formed when metal M was added to dilute sulphuric acid?
(1)
(ii) Describe a test, and the result you would expect, for this gas.
(2)
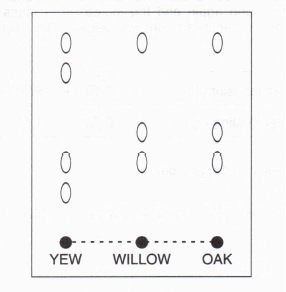
6. A teacher asked the class to investigate whether the green colour of the leaves of three species of tree in the school grounds (oak, yew and willow) was due to the same substances. The class was told that paper chromatography, with propanone as the solvent, could be used to do this. The teacher gave each pupil an extract of the colouring matter from the leaves of each tree.
(a) How might the teacher have extracted the green colouring matter from the leaves?
(2)
(b) Describe how you would carry out the paper chromatography experiment to find out whether the green colour was due to the same substances.
(5)
(c) The result of John's chromatography experiment is shown below. What conclusions can you make about the coloured substances in the three different leaves?
(3)
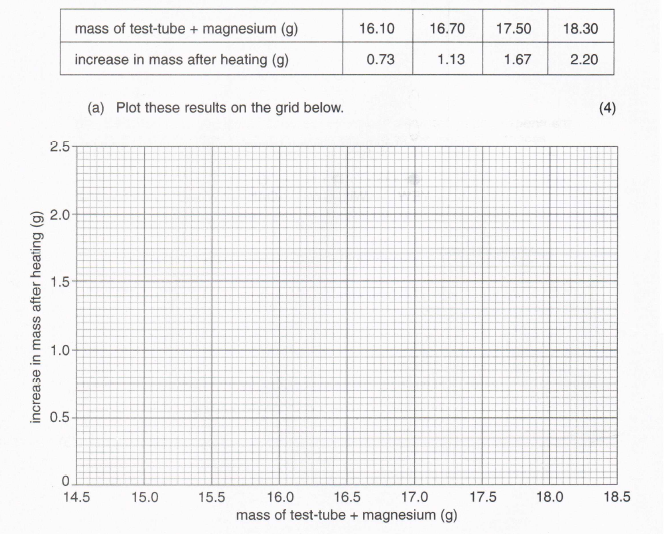
7. Sally and three friends did an experiment to investigate the change in mass which occurs when magnesium is heated. Each one of them found the mass of a test-tube containing a sample of magnesium and then heated the magnesium until no further reaction took place. The tubes and contents were allowed to cool, their masses found again and the increases in mass calculated. The results of their experiments are shown below. (The test-tubes were all identical.)
(b) How does the increase in mass depend on the mass of magnesium heated?
(1)
(c) (i) Explain how the mass of the identical test-tubes used in the experiments can be obtained from the graph.
(2)
(ii) Find the mass of the test-tube.
(2)
(iii) What increase in mass occurs when 2.0 grams of magnesium are heated?
(1)
(d) Sally was unsure whether all of her magnesium had reacted. Describe what she could do to check whether it had all' reacted.
(2)
(e) (i) Write a word equation for the reaction which took place.
(1)
(ii) What type of reaction is this?
(1)
(f) Describe how you could obtain a sample of magnesium sulphate crystals from the contents of the test-tubes after heating, given a supply of dilute sulphuric acid.
(4)