1. a) The method used to get salt from sea water is called
(1)
b) An example of a metal is
carbon iodine sulphur zinc
(1)
c) The most reactive metal in the list below is
calcium gold iron mercury
(1)
d) An example of a compound is
air graphite hydrogen water
(1)
e) The commonest gas in air is
hydrogen carbon dioxide nitrogen oxygen
(1)
2. Choose a gas from the list below to complete each sentence correctly.
carbon dioxide carbon monoxide hydrogen
nitrogen oxygen sulphur dioxide
(a) pollutes the air in some towns and causes acid rain. (1)
(b) dissolves in rain anywhere and makes it very slightly acidic. (1)
(c) supports combustion. (1)
(d) is produced when carbon burns in a limited air supply. (1)
(e) will relight a glowing splint. (1)
3. Underline the fuels in this list.
heat oxygen petrol sunlight water wood
(2)
4. Use a word from the list below to complete each sentence correctly.
boils condenses evaporates freezes melts sublimes
(a) When ice is heated, it to form a liquid.
(b) When solid carbon dioxide (dry ice) is heated, it to form a gas.
(c) When pure water reaches 100°C, it
(d) Steam on a cold surface to form tiny water droplets.
(4)
5. Complete the following word equations.
(a) hydrated copper sulphate ---(heat)---> anhydrous copper sulphate + (1)
(b) iron oxide + ---(heat)---> magnesium oxide + iron (1)
(c) potassium + oxygen ---> (1)
(d) From the chemicals named in parts (a), (b) and (c), write down
(i) a compound (1)
(ii) a metallic element (1)
(iii) a metal that is being oxidised in a reaction (1)
(iv) an oxide which dissolves in water to give an alkaline solution
(1)
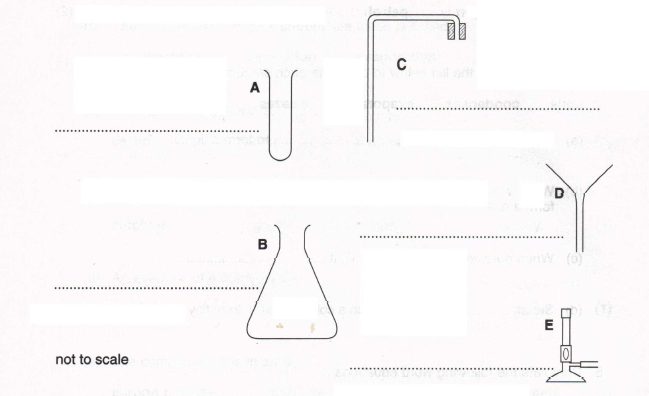
6. (a) Name the pieces of apparatus shown below.
(A)
(B)
(C)
(D)
(E) (5)
(b) Select the pieces of apparatus you would use to
(i) distil water from ink
(ii) remove sand from a mixture of sand and sea water.
(5)
(c) Which additional material would you need in (b) (ii)?
(1)
7. Several parts of the body can be replaced with artificial alternatives.
(a) (i) Suggest why some replacement parts used for joints are made from metals.
(1)
(ii) Suggest a suitable metal and explain why you have chosen it.
(1)
Metal: (1)
Reason: (2)
(b) Suggest why replacement heart valves are usually made from plastic.
(1)
(c) Which material would you choose for artificial teeth, and why?
Material: (1)
Reason: (2)
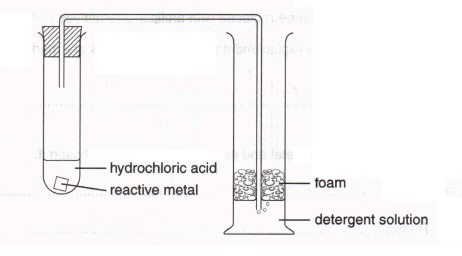
8.
Using the apparatus shown above, the gas produced during the reaction causes bubbles of foam to appear in the measuring cylinder.
(a) (i) Which gas is being produced?
(1)
(ii) How would you test for this gas?
(1)
(b) Suggest how you could use this apparatus to compare the reactivity of three different metals.
(3)
(c) Which three things would you need to keep constant to make sure the comparison was fair?
1. (1)
2. (1)
3. (1)
9.
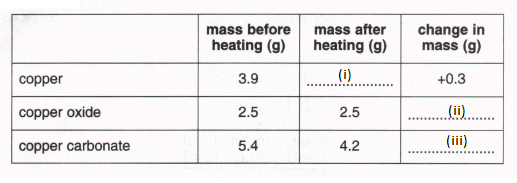
(a) Fill in the blank spaces in the table which shows the effect of heating three substances in air.
(i) (ii) (iii) (3)
(b) Which substance has been oxldised?
(1)
(c) Which substance has been decomposed by heating?
(1)
(d) Using one of the three substances in a test tube connected to a gas syringe, describe how you would use this effect of heating to find the density in the air of either oxygen or carbon dioxide. You should include a labelled diagram of the apparatus.
(5)