1 a) A substance having a fixed volume and which flows easily is a
b) A substance in which the particles are moving rapidly and are widely separated is a
crystal gas liquid solid
c) The best way to obtain salt crystals from sea water is by
chromatography distillation evaporation filtration
d) Ethanol can be obtained from wine by
chromatography distillation fermentation filtration
e) An element which is shiny and is attracted to a magnet might be
carbon copper iron magnesium
f) A liquid which freezes at 0 °C and which turns solid anhydrous copper sulphate blue is
ethanol petrol sea water water
g) When copper sulphate is dissolved in water, the water is the
filtrate solute solution solvent
h) When solid iodine is heated rapidly it can change straight into a gas. The iodine has
boiled condensed melted sublimed
i) A Bunsen burner flame is hottest when the gas tap is
fully open and the air hole is closed half open and the air hole is closed
fully open and the air hole is open half open and the air hole is open
(9)
2. Complete the word equations for
(a) the reaction between iron oxide and carbon
iron oxide + carbon --> + (2)
(b) the decomposition of calcium carbonate on heating
calcium carbonate --> + (2)
3. Name a substance which when heated in air would
(a) be unchanged chemically
(1)
(b) increase in mass
(1)
(c) give off oxygen.
(1)
4. A burning candle is covered with a gas jar. It burns for a while and then goes out. A little condensation forms on the sides of the jar.
(a) Which gas in the air enables the candle to burn?
(1)
(b) Why does the candle go out?
(1)
(c) Which gas, normally present in the air in a small amount, is formed during burning?
(1)
(d) Explain the formation of condensation on the sides of the jar.
(2)
(e) Which elements are combined in candle wax?
(2)
5. (a) Lead is sometimes used as a roof covering.
(i) Give a chemical property of lead which makes it a good covering for a roof.
(1)
(ii) Give a physical property of lead which makes it a good covering for a roof.
(1)
(b) Lead is no longer used for water pipes. Why not?
(1)
(c) Pencil leads are not really lead at all.
(i) Which element do they contain?
(1)
(ii) Which property makes this element useful for pencil leads?
(1)
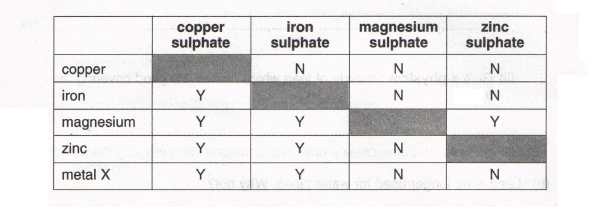
6. John decided to investigate the reactivities of various metals. He added samples of each metal to solutions in water of the sulphates of some other metals. His results are shown in the table. Y indicates that he observed a reaction, N indicates that he observed no reaction.
(a) (i) Describe what you would expect to see when plenty of powdered zinc is added to copper sulphate solution.
(2)
(ii) Complete the word equation for the reaction
zinc + copper sulphate --> + (2)
(b) (i) Arrange the metals, copper, iron, magnesium, zinc and X in order of reactivity (most reactive first).
(2)
(ii) Describe and explain what you would expect to see when metal X is added to dilute sulphuric acid.
(3)
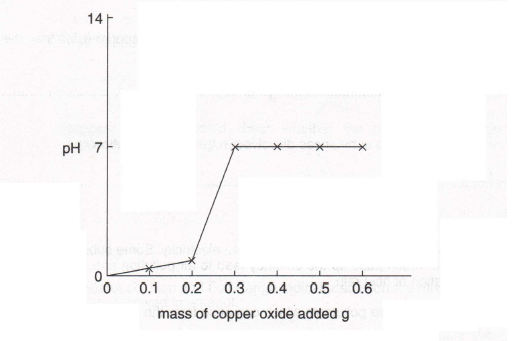
7. Lucy was carrying out some experiments on pH. One of her experiments involved adding portions of copper oxide powder to 100 ern" of dilute hydrochloric acid and measuring the pH of the solution after each addition. Here is a graph of her results.
(a) What other readings might Lucy have taken to try to achieve a smoother graph?
(1)
(b) How could she have measured the pH?
(1)
(c) (i) According to the graph, what mass of copper oxide had to be added to the hydrochloric acid to give a neutral solution?
(1)
(ii) Suggest why the pH did not change when more copper oxide than this was added.
(1)
(iii) Name the substances dissolved in the neutral solution.
(2)
8. Coal is burnt in power stations to generate electricity. Some substances in the flue gases which pass up the chimney lead to air pollution and, eventually, to the formation of acid rain.
(a) (i) Name two pollutants which can arise from the burning of fossil fuels.
1. (1)
2. (1)
(ii) Name one pollutant which causes acid rain.
(1)
(iii) Give one effect of acid rain.
(1)
(b) One suggested way of reducing the formation of acid rain is to neutralise the pollutant in the flue gases which is responsible for its formation.
(i) What is meant by 'neutralise'?
(2)
(ii) Name a substance through which the flue gases might be passed to neutralise and remove the pollutant.
(1)
Explain why this particular substance can be used.
(1)
Suggest how you could check whether the pollutant had been removed from the flue gases.
(2)
9. Universal Indicator solution and Full Range Indicator solution are mixtures of a number of dyes dissolved in ethanol.
(a) Describe how you would use chromatography to find out how many dyes were in each mixture. Draw a diagram if you wish.
(5)
(b) How would you tell whether the same dyes were present in both indicators?
(2)