1. a) When lighting a Bunsen burner, the air-hole should be
b) An example of an alkali is
lemon juice limewater salt water vinegar
c) A method used to get pure water from sea water is called
condensation distillation evaporation filtration
d) When a piece of marble or limestone is heated strongly, the gas given off is
carbon dioxide hydrogen oxygen sulphur dioxide
e) A gas which causes acid rain is
hydrogen methane nitrogen sulphur dioxide
f) The pH of vinegar is about
3 7 10 13
g) An example of a metal is
carbon iodine sulphur zinc
h) The most reactive metal in the list below is
calcium gold iron mercury
i) An example of a compound is
air graphite hydrogen water
j) The commonest gas in air is
carbon dioxide hydrogen nitrogen oxygen
(10)
2. It was a cold day, so Fred hadn't opened his kitchen window. After a while, he could no longer see through the window.
(a) What had happened to prevent Fred from seeing through the window?
(1)
(b) Using a 'o' to represent a water molecule, show the arrangements of these molecules ln the saucepan and in the air.

3. aluminium calcium copper iron silver zinc
From this list choose one metal which can be used for
(a) making water pipes (1)
(b) making window frames (1)
(c) making a magnet (1)
(d) reacting with cold water, to release hydrogen gas (1)
(e) galvanising iron to stop it rusting (1)
4. Draw a diagram to show a Liebig condenser in use. Label clearly the other pieces of apparatus being used with the condenser.
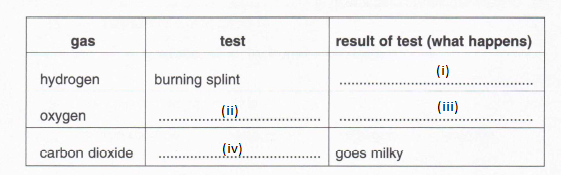
5. The table below shows the tests which can be used to identify different gases.
Fill in the missing spaces.
(i) (1)
(ii) (1)
(iii) (1)
(iv) (1)
6. Fred was hungry. He boiled an egg on his gas cooker. First he put some cold water from the tap into a saucepan and lit the gas. He noticed that the outside of the saucepan became wet almost immediately.
(a) What was the liquid on the outside of the saucepan?
(1)
(b) How was this liquid produced?
(1)
(c) Describe a chemical test for the liquid you have given in part (a).
(2)
7. When the water was bubbling, Fred put an egg into the water and cooked it.
(a) What is the boiling-point of pure water? (1)
(b) Suggest why the boiling-point of the water in Fred's saucepan was higher than that of pure water.
(1)
8. Fred's saucepan was made of the metal aluminium.
(a) Give two reasons why metals rather than non-metals are used to make saucepans.
(i) (1)
(ii) (1)
(b) Why are plastics not suitable for making saucepans, although they are used for buckets and washing-up bowls?
(2)
9. Fred was still hungry! He therefore ate a packet of salted crisps. While he was doing this, he wondered how he might separate the salt from the crisps. He emptied a second packet of crisps into a bowl of water.
(a) Why should Fred have crushed the crisps before adding them to the water?
(1)
(b) What should Fred do next to obtain dry salt from the crisps? Give a reason for each part of your answer. You may find it helpful to draw some diagrams on the opposite page.
(6)
(c) (i) Fred had a third packet of crisps labelled 'Salt and Vinegar flavoured'.
Suggest another experiment which he might attempt in order to obtain a small quantity of vinegar from the crisps.
(3)
(ii) Give two reasons why this experiment might not be successful.
(2)
10. Fred then ate some stewed blackberry and apple. The stewed blackberry and apple was a reddish-purple colour. However, when Fred did his washing up, using a washing-up liquid which was mildly alkaline, the colour of the juice became much bluer.
(a) Suggest why the colour changed.
(2)
(b) What is the name given to substances which change colour in this way?
(1)
(c) What colour might the blackberry and apple juice be when mixed with the following substances from Fred's kitchen?
(i) vinegar (1)
(ii) salt (sodium chloride) (1)
(iii) lemonade (1)
(iv) Milk of Magnesia indigestion medicine (1)
Fred had cooked his blackberry and apple in a copper saucepan. When he washed it up, the copper inside was much brighter and shinier where the fruit had been.
(d) Suggest an explanation for this observation.
(2)