1. a) When copper reacts with sulphur, the product is
b) The pH value of a strong alkali could be
1 4 7 13
c) When an acid reacts with limestone, one of the products is
carbon dioxide hydrogen nitrogen oxygen
d) When an acid reacts with an alkali, one of the products is
an element a filtrate a salt a sublimate
e) Copper foil, hydrogen gas, liquid nitrogen and solid zinc are
all compounds all elements all mixtures some elements and some compounds
f) When sulphur reacts with air to give sulphur dioxide, the reaction can be described as
combustion condensation decomposition neutralisation
(6)
2. Ethanol boils at 78°C and water at 100°C. A pupil distilled a mixture of the two liquids and tested the distillate when the temperature was 79°C. This liquid was flammable and the cobalt chloride paper test showed that some water was present. The pupil then carried on heating and tested the distillate at 95 °C, when water was present, but the liquid was not flammable.
(a) Draw a diagram of the apparatus you would use for the distillation, labelling
(i) the source of heat
(ii) the thermometer
(iii) the Liebig condenser
(iv) the direction of flow of water in the condenser
(6)
(b) How would you use cobalt chloride paper to show that water is present?
(2)
(c) Why was the distillate at 79°C flammable?
(2)
(d) Why was the distillate at 95°C not flammable?
(1)
(e) What would you expect the distillate to be at 100°C?
(1)
3. Here are five gases: argon; carbon dioxide; hydrogen; nitrogen; oxygen.
(Each can be used once, more than once or not at all in your answers.) Which one is formed when
(a) potassium manganate(VII) is heated (1)
(b) copper carbonate is heated (1)
(c) copper oxide reacts with carbon (1)
(d) photosynthesis takes place (1)
(e) zinc reacts with hydrochloric acid (1)
(f) petrol burns? (1)
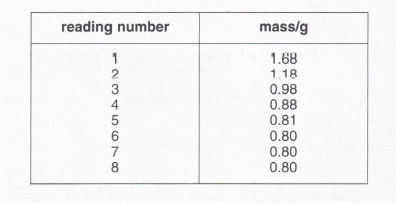
4. When solid magnesium carbonate is heated, it gives off carbon dioxide and leaves solid magnesium oxide behind. In an experiment, James took a testtube containing some magnesium carbonate and weighed it. He then heated the test-tube for about a minute, allowed it to cool and reweighed it. After repeating the heating and weighing seven more times, he obtained the following readings. (The mass of the empty test-tube has been subtracted from each reading.)
(a) Plot the readings on the graph paper below and draw the best fit line through them.
(5)
(b) Why were readings 6, 7 and 8 identical?
(1)
(c) What mass of carbon dioxide was given off in this experiment? Show your working.
(2)
(d) The last balance reading was actually 15.58 g. What was the mass of the test-tube?
(1)
(e) How would you show that the gas given off in this experiment was carbon dioxide?
(3)
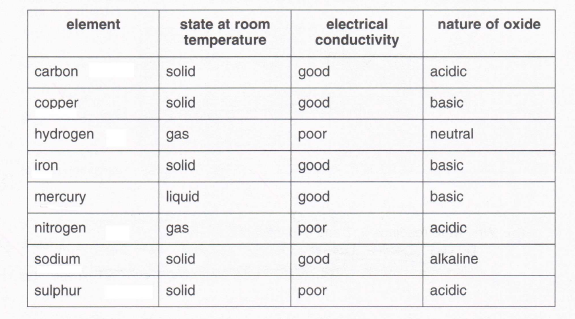
5. The table shows the properties of some elements and their oxides.
Use the information in the table to say whether the following statements are true or false, giving reasons for your answers.
(a) Metals are good conductors of electricity.
(i) The statement is:
(ii) Reasons: (3)
(b) At room temperature, non-metals are liquids or gases.
(i) The statement is:
(ii) Reasons: (3)
(c) Non-metal oxides are acidic.
(i) The statement is:
(ii) Reasons: (2)
(d) Metal oxides are basic or alkaline.
(i) The statement is:
(ii) Reasons: (2)
6. Chromatography, distillation, evaporation, filtration and sublimation are all used in laboratories. Which can be used to
(a) obtain pure water from seawater (1)
(b) separate the dyes in Universal Indicator (1)
(c) dry some wet salt crystals (1)
(d) separate the sand from a mixture of sand and water (1)
(e) obtain liquid oxygen from liquid air? (1)
7. A wet piece of iron wool was wedged at the bottom of a test-tube. The test-tube was then turned upside down with its open end dipping into a beaker of water. After a day the iron wool had gone rusty and the water had risen one fifth of the way up the tube.
(a) Draw labelled diagrams of the experiment (i) at the start and (ii) after one day.
(4)
(b) (i) Why did the water rise up the test-tube?
(2)
(ii) Why did the water rise only one fifth of the way up the test-tube?
(2)
(iii) What would a burning splint do in the gas left in the test-tube?
(1)